Symbols Glossary Definition
Symbols Derived from Standards
| Symbol | Standard Reference | Standard Title | Symbol Title | Explanatory Text |
|---|---|---|---|---|

| ASTM F2503-20 | Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment | MR Safe | An item which poses no known health hazards from exposure to any MR environment. |

| ASTM F2503-20 | Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment | MR Conditional | An item with demonstrated safety in the MR environment within defined conditions. |

| ISO 15223-1, Reference 5.1.1 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Manufacturer | Indicates the medical device manufacturer. |

| ISO 15223-1, Reference 5.1.2 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Authorized representative in the European Community | Indicates the Authorized representative in the European Community. |

| ISO 15223-1, Reference 5.1.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Date of manufacture | Indicates the date when the medical device was manufactured. |

| ISO 15223-1, Reference 5.1.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Use-by date | Indicates the date after which the medical device is not to be used. |

| ISO 15223-1, Reference 5.1.5 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Batch code | Indicates the manufacturer's batch code so that the batch or lot can be identified. |

| ISO 15223-1, Reference 5.1.6 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Catalogue number | Indicates the manufacturer's catalogue number so that the medical device can be identified. |

| ISO 15223-1, Reference 5.1.7 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Serial number | Indicates the manufacturer's serial number so that a specific medical device can be identified. |

| ISO 15223-1, Reference 5.2.1 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Sterile | Indicates a medical device that has been subjected to a sterilization process. |

| ISO 15223-1, Reference 5.2.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. |

| ISO 15223-1, Reference 5.2.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. |
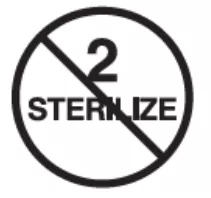
| ISO 15223-1, Reference 5.2.6 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Do not resterilize | Indicates a medical device that is not to be resterilized. |

| ISO 15223-1, Reference 5.2.7 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Non-sterile | Indicates a medical device that has not been subjected to a sterilization process. |

| ISO 15223-1, Reference 5.2.8 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Do not use if package is damaged | Indicates a medical device that should not be used if the package has been damaged or opened. |
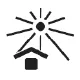
| ISO 15223-1, Reference 5.3.2 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Keep away from sunlight | Indicates a medical device that needs protection from light sources. |

| ISO 15223-1, Reference 5.3.5 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Lower limit of temperature | Indicates the lower limit of temperature to which the medical device can be safely exposed. |

| ISO 15223-1, Reference 5.3.6 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Upper limit of temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed. |
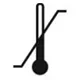
| ISO 15223-1, Reference 5.3.7 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed. |

| ISO 15223-1, Reference 5.4.2 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Do not reuse | Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. |

| ISO 15223-1, Reference 5.4.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Consult instructions for use | Indicates the need for the user to consult the instructions for use. |

| ISO 15223-1, Reference 5.4.3 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Consult electronic instructions for use | Indicates the need for the user to consult the electronic instructions for use (eIFU) at the specified website location. |

| ISO 15223-1, Reference 5.4.4 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Caution | Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. |

| ISO 15223-1, Reference 5.4.5 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Contains or presence of natural rubber latex | Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device. |
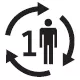
| ISO 15223-1, Reference 5.4.12 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Single patient - multiple use | Indicates a medical device that may be used multiple times (multiple procedures) on a single patient. |

| ISO 15223-1, Reference 5.7.1 | Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements. | Patient number | Indicates a unique number associated with an individual patient. |

| ISO 7010, Reference W021 | Graphical symbols — Safety colours and safety signs — Registered safety signs | Warning; Flammable material | To warn of flammable material. |

| ISO 7010, Reference W023 | Graphical symbols — Safety colours and safety signs — Registered safety signs | Warning; Corrosive substance | To warn of corrosive substance. |
Symbols Not Derived from Standards
| Symbol | Standard Reference | Standard Title | Symbol Title | Explanatory Text |
|---|---|---|---|---|
Rx Only | 21 CFR 801.109 | Labeling; Prescription devices | Prescription use only | Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. |
| MDD 93/42/EEC Annex II; MDR 2017/745 Annex V | The requirements for accreditation and market surveillance relating to the marketing of products; Medical Device Directive and Medical Device Regulation. | European conformity | European conformity (CE) mark for Class I medical devices. |

| MDD 93/42/EEC Annex II; MDR 2017/745 Annex V | The requirements for accreditation and market surveillance relating to the marketing of products; Medical Device Directive and Medical Device Regulation. | European conformity | European conformity (CE) mark with Notified Body identification number for Class Im, Ir, Is, IIa, IIb, III medical devices Notified Body No. 0086: BSI, United Kingdom |

| MDD 93/42/EEC Annex II; MDR 2017/745 Annex V | The requirements for accreditation and market surveillance relating to the marketing of products; Medical Device Directive and Medical Device Regulation. | European conformity | European conformity (CE) mark with Notified Body identification number for Class Im, Ir, Is, IIa, IIb, III medical devices Notified Body No. 2797: BSI, Netherlands |

| MDR 2017/745 Annex 1 23.2(q) | The requirements for indicating that a device is a medical device; Medical Device Regulation. | Medical device | Indicates that the device is a medical device. |

| N/A | N/A | Telephone receiver or handset | Indicates the telephone contact number. |

| Medicines and Medical Device Act 2021 | Part 4, Chapter 1, Section 16 (1) (f) | UKCA marking | Signifies Great Britain technical conformity. |